Research
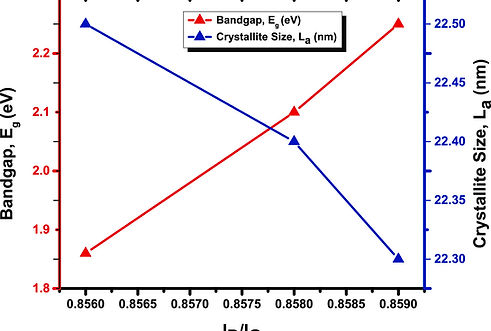
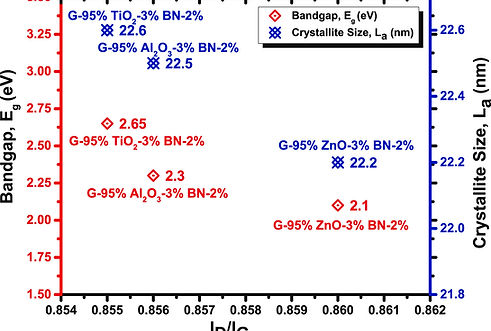
The introduction of boron nitride (BN), zinc oxide (ZnO), aluminum oxide (Al2O3), and titanium oxide (TiO2) as dopants in pristine graphene leads to the modification of its transport properties, resulting in the production of graphene semiconductors and alteration of its semiconductive characteristics. The achievement of high-quality electronic devices necessitates considering doping and producing a broader energy bandgap in graphene. The optical determination of charge density in intrinsic graphene may be achieved by utilizing the D Raman peak, where an increased charge density is associated with a decreasing peak split. Strong connections have been seen between the energy bandgap (Eg) and the locations of the G and D peaks. Various doping materials exhibit distinct variations and effects on the doping process of graphene. The determination of doping levels in graphene may be achieved with far greater accuracy by observing the ID/IG ratios, compared to the traditional method of measuring the G-band shift with charge. The determination of average crystallite size, as well as the observation of the parameter La, is crucial in comprehending the doping and flaws present in graphene materials that have been manufactured.
In the realm of photovoltaic-thermal (PVT) systems, optimizing operating temperatures for photovoltaic (PV) panels is a challenge. This study introduces a novel solution: a sprayed water PVT system that simultaneously harnesses energy and electricity. The aim is twofold: generate electricity through PV panels and produce hot water via a flat plate collector, using an innovative cooling mechanism. Water sprayed onto the PV panel's surface flows to a collector for storage. With varied flow rates, optimal panel efficiency occurs at a 45⁰ tilt angle, accompanied by lower collector outlet temperatures at higher flow rates. The collector achieves a peak thermal efficiency of 70.6 %, producing hot water at 84.6 °C. Notably, a significant PV panel efficiency enhancement, up to 16.78 %, especially at 1.56 L/min flow rate, is observed. The cooling technique consistently reduces panel temperatures from 45.08 °C to 34.12 °C. A self-cleaning spray mechanism improves efficiency by 2.53 %, resulting in an overall system efficiency of 83.3 %. This research offers an innovative approach to enhance energy generation and electricity in PVT systems, promising sustainable energy optimization.


Nanoparticles (NPs) are synthesized in different methods such as physical or chemical which are costly and harmful to the environment. However, green synthesis is a cost-effective and environment-friendly NPs synthesis approach. Green-synthesized NPs are highly effective against various bacterial strains. Zirconium dioxide nanoparticles (ZrO2NPsZrO2NPs) have been synthesized in this research using a cost-effective green synthesis approach for improved antimicrobial activity. For the synthesis of the NPs, Alocasia indica has been used as a reducing agent. The extract of Alocasia indica was prepared and reacted with the light solution of ZrOCl2 ⋅ 8H2O. The successful formation of ZrO2NPsZrO2NPs was confirmed by the color change in the solution. Some analytical techniques, including Ultraviolet-visible (UV) spectroscopy, Fourier Transformed Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive X-ray (EDX), X-ray diffraction (XRD), and bacterial inhibition analyses have been performed to analyze the performance of the synthesized NPs. The peaks formed in the UV analysis also confirmed the formation of NPs in the solution. The presence of various functional groups in the synthesized ZrO2NPsZrO2NPs has been confirmed by the FTIR analysis. TEM analysis revealed that the synthesized NPs were almost circular. The formation of peak confirmed the crystalline structure of the NPs. Impressively; a 99.99% bacterial inhibition rate was obtained against the gram-positive bacteria strain. The synthesized ZrO2NPsZrO2NPs have great potential for biomedical applications such as medicine and implants for its excellent antimicrobial applications.


Excellent characteristics and a wide range of applications made nanotechnology a key focus for modern researchers. However, environment-friendly and cost-effective measures still need to be taken to synthesize nanoparticles. This research focused on synthesizing Green Ceramic-based Nanoparticles (GCNPs) using the sol-gel method. This method is environment-friendly and cost-effective. Different analytical techniques were employed to characterize the obtained GCNPs. Crystallographic parameters were obtained through the X-ray diffraction (XRD) analysis. Morphological analysis was performed by Field Emission Scanning Electron Microscope (FESEM) analysis. The presence of different elements was identified by elemental analysis (EDX). Various functional groups present in the GCNPs were identified by Fourier Fourier-transformed infrared Spectroscopy (FTIR) analysis. XPS and zeta potential analyses composition characterization and surface charge stability, respectively. Antibacterial strains against E. coli were performed. The FTIR analysis confirmed the presence of different organic compounds in the synthesized GCNPs which helped to increase bacterial inhibition in the antibacterial test. The FESEM images confirmed irregularity in the shape and aggregation of the synthesized GCNPs. The minimum average particle size was obtained from sample S2 which is 376.35 nm. EDX analysis confirmed the presence of silicon particles in the GCNPs. The crystalline nature of the GCNPs was assured by XRD analysis. The silicon percentages were higher than the other atoms shown by the XPS analysis. A significant amount of bacterial inhibition which was 18.0 mm obtained from sample S3. Finally, GCNPs were prepared, characterised, and significantly applied for diverse implementation for the safety of the healthcare field on a broad scale.







